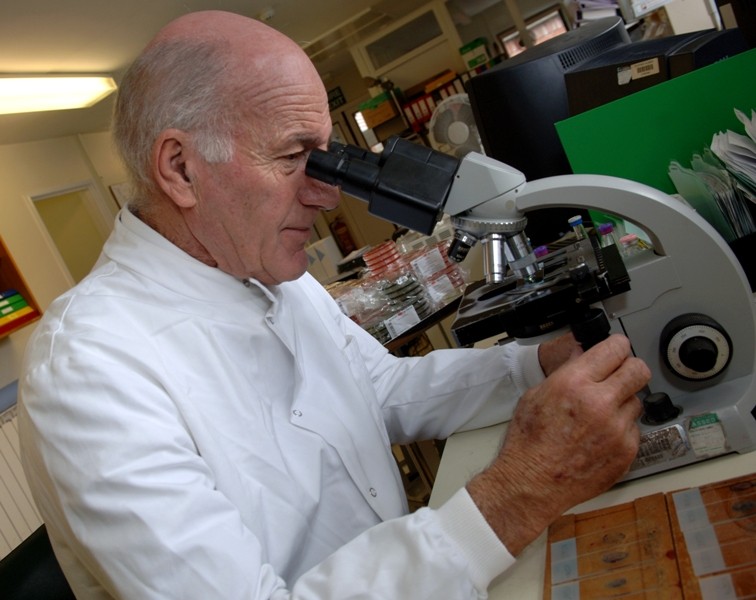
At South Warwickshire University NHS Foundation Trust, we are committed to supporting and operating a dynamic programme of research to pave the way for new treatment approaches and contribute to providing the best care for patients.
Research is a vital means of developing these new approaches to patient treatment and care for a wide range of illnesses and conditions. It allows us to assess the efficacy of new medicines, treatments and diagnostic regimes, providing evidence we can use to benefit patients. We operate an evolving programme of research studies that have great potential to create long-term improvements for patients everywhere. Through our research studies, the team can also deliver immediate improvements for the patients who have volunteered to participate in them.
We currently undertake a wide range of studies ranging from simple questionnaires to drug trials at Warwick Hospital, Central England Rehabilitation Unit - Royal Leamington Spa, Stratford Hospital and across our Community Services. Patients may be invited to participate in a study while being treated at the hospital.
Our Research team also works closely with national and local organisations concerned with developing these new approaches to patient treatment and care.
Contact details:
Location: Research and Development Office, 1st Floor, Building 3, Saltisford Office Park, Ansell Way, Warwick CV34 4UL
Email: Research
Telephone: 01926 495321 ext. 8107
What are we working on at the moment?
We currently have around 40 research studies open. Click the tabs below to find out more information. A number of these are focused on developing new approaches to treating certain types of cancer.
Research could not happen without the support of patients, carers and members of the public. Your help to assist us improve services is vital, both for the present and future generations. Each year about 750 new patients take part in our studies and we currently have approximately 40 studies open.
Taking part in a trial may include any of the following:
- Talking to researchers
- Completing questionnaires
- Letting us take blood samples or other measurements e.g. blood pressure, urine tests
- Taking a new medicine or trying a new treatment
Why take part?
- You may benefit from a new treatment or test or others may benefit in the future
- You may want to contribute to improving care for others
- You may feel that you like the extra attention and time that sometimes participation in a trial can provide.
- You may want to increase your knowledge about your condition.
Research Studies
Research Studies that we are currently involved in include the following specialities:
- Diabetes
- Critical Care
- Cancer and haematology
- Heart disease
- Surgery
- Midwifery
- Emergency Medicine
- Gastroenterology
- Trauma and Orthopaedics
- Paediatrics
- Rheumatology
We support clinicians to open studies and actively seek to open studies in new specialities. Participation as a patient is voluntary and patients who do not wish to participate will still receive the best care that we can offer.
All research conducted within the Trust has been reviewed by the Research Governance department within the Research and Development team, to ensure robust standards are maintained.
Find out what you need to know about Research and Personal Data.
Should you wish to know more about the studies open to recruitment within any particular specialty please email research
SWFT is one of the Partnership Trusts for the Research and Recovery Delivery Network (RRDN).
South Warwickshire University Foundation Trust (SWFT) and the University of Warwick (UoW) are developing closer working relationships particularly in the field of academic and clinical research.
SWFT has a five-year research strategy. This research strategy is aligned to SWFT and Foundation Group’s values, including inclusivity, long-term ambitions and strategy.
The research strategy will focus on the needs of SWFT’s local community, role as a lead provider within the Coventry and Warwickshire Integrated Care Partnership and ambition to be able to apply for membership of the University Hospital Association.
To deliver this strategy SWFT will partner with the UoW. This will build upon established collaborations between a combined acute and community trust rated as outstanding by the Care Quality Commission (CQC) and internationally recognised centres of excellence at the University of Warwick.
SWFT will continue to collaborate with other local and national research partners, including Coventry University, the Applied Research Collaboration (ARC) West Midlands and the National Institute for Healthcare Research (NIHR).
SWFT’s research agenda will focus primarily on 6 identified work streams:
- Recruitment into local and national NIHR studies
- Establishing a Clinical Trials Unit to support data analysis for clinical studies including the development of clinical models regarding service delivery to support improvements in efficiency and cost effectiveness
- A basic science research collaborative that will enable researchers to collect clinical material for laboratory studies from our hospital group population
- A primary care collaborative that will investigate the management of chronic health conditions and the use of digital technology in primary care.
- A digital collaborative that will focus on the use of technology to support the management of chronic conditions, including the use of telemedicine
- A local partnership collaborative to focus on the care of patients with chronic health conditions across boundaries in primary care, secondary care, social services and the voluntary sector.
This will require recurrent funding, the establishment of a research faculty, a research delivery group, managerial support and joint appointments with local universities.
This summary is based on papers approved at SWFT trust Board and Management Board.
- To see if your idea is research.
- For research planning.
- For the approval process.
- For roles and responsibilities.
Please contact research
SWFT Staff** When undertaking research, staff must adhere to the Trust's procedure in line with the Research Governance Framework.
GEH SWFT Knowledge and Library Service team are proud to help support colleagues undertaking research at both our NHS Trusts. Whether you are an experienced researcher or just starting the research project for your Apprenticeship, foundation degree or Masters, we are on site and online to help. The Research webpage on the library website is a good starting point but please don’t hesitate to contact any of our team. Here’s just some of the ways we can help:
- Information Skills training
We provide regular group sessions on how to plan and do an effective literature search. Visit our training webpages to view details of our next sessions. Our Clinical Librarians can also provide 1:1 advice on refining search strategies, database selection and signpost you to further reading to support your research. Contact us.
- Critical appraisal training
Why not book on one of our regular Critical Appraisal training sessions to develop or practise your skills in evaluating research? More information and future dates.
- Access to major research databases
Visit the NHS Knowledge and Library Service Hub where you can find links to clinical and health management databases and other useful resources such Grey Literature resources and Cochrane Library.
- Current awareness
We can help you keep up to date in your area of research. Sign up to receive personalised email bulletins from KnowledgeShare.
- Books for researchers
The Library collection includes books on all aspects of research from doing a literature or systematic review to critical appraisal and research methods. Search our regional library catalogue HeLM here.
- Article request service
Having trouble finding the full text of an article? Click here for handy search tips or complete our online request form
Writing up Research for Publication
Ellis P. British Journal of Healthcare Assistants 2023;17(10):380-384.
Writing for publication is a daunting task for many people, but it need not be. Writing up a piece of research for publication should be the pinnacle of the research process, the point at which the researcher gets to share their work with a wider audience.
This paper explains wat needs to go into a research paper that is being prepared for publication. It offers a step-by-step guide to the process to help the novice and more experienced researcher structure their research paper. This paper covers writing up both quantitative and qualitative research and examples, where used, may be drawn from either or both paradigms. This paper does not cover what is needed to publish a review or opinion piece.
Article available to view online with an NHS OpenAthens password. No OpenAthens account? Register for your free account.
The Trust works with the National Institute for Health Research (NIHR) West Midlands Regional Research Delivery Network, which gives researchers and delivery teams the practical support they need in our region so that more research takes place and more people can take part. Find out more on the Network's website. To find opportunities to take part in research, click here.

Research Governance
Associate Medical Director of Research
Head of Research & Development
Email: Research
Central Research
Lead Research Nurse
Email: CentralResearchTeam
Cancer Research
Rigby Senior Research Nurse
Email: cancerresearch
Maternity Research
Research Midwife
Email: ResearchMidwife
Follicular Lymphoma (FL) and Marginal Zone Lymphoma (MZL) are indolent (slow growing) incurable blood cancers in the Non-Hodgkin Lymphoma group. Approximately 5,000 people in the UK are diagnosed with FL or MZL each year. First-line chemo-immunotherapy (the first treatment a patient receives) is very effective, but the cancer relapses (comes back) in almost all cases and the duration of response (how long it takes to come back) gets shorter with each new treatment. Disease relapse is the cause of death in most patients.
OLYMPIA-5 is a commercial clinical trial by Regeneron investigating odronextamab, an ‘anti-CD20 x anti-CD3 bispecific antibody’ that helps the body’s immune system target and kill the cancer cells in FL and MZL.
The trial is recruiting adults with FL or MZL that is refractory (has not responded to treatment) or has relapsed (come back). The aim of the trial is to determine whether odronextamab is better than an existing drug, rituximab, when used in combination with lenalidomide.
Acute Myeloid Leukaemia (AML) is a blood cancer characterised by the rapid growth of abnormal cells that interfere with normal blood cell production. The treatment for AML is extremely challenging, and patients often feel fatigued (very tired), have low mood, have poor nutrition, and are unable to do any physical activity.
‘Prehabilitation’ is a programme of support designed to improve a patient’s overall health and fitness before major treatment. AML patients often present as a medical emergency, starting treatment within days of diagnosis. This leaves little opportunity for conventional prehabilitation and therefore it is not routinely offered to people with AML in the UK.
PROPEL is asking whether remote personalised emotional, nutritional, and physical support for patients with AML, and the related condition Myelodysplastic Syndrome with Excess Blasts (MDS-EB2), can reduce fatigue, help patients get through all the cycles of intensive chemotherapy and stem cell transplant (if needed), and improve clinical outcomes.
Myeloma XV (RADAR) is recruiting patients newly diagnosed with Multiple Myeloma (MM) who are eligible for a stem cell transplant. MM, also known as Myeloma, is a cancer that affects the bone marrow (spongy tissue found inside some bones that produces blood cells). Over 5,500 patients are diagnosed with MM each year in the UK. MM is an incurable relapsing-remitting cancer, meaning there are periods where the disease is active (relapsing) and requires treating, followed by periods where treatment may not be required (remitting).
Significant progress has been made in developing new lines of treatment over the past 15 years. Newly diagnosed patients now survive on average 5-6 years, with 37% of men and 28% of women surviving 10 years. Currently all MM patients are given the same treatment, this UK-based Phase III study is investigating the personalisation of MM care. Some patients will have genetic markers that suggest their cancer will be more difficult to treat. These higher-risk patients, along with those whose cancer has not responded well to initial treatment, are offered more intensive therapy to extend the remitting period, while lower risk patients can receive reduced treatment with the aim of reducing side effects.
We are also recruiting to the following trials: COSMOS (smouldering myeloma), Cutaneous Tumour (squamous cell carcinoma and keratoacanthoma), CVLP (cancer vaccines), FOxTROT 2 & 3 (colorectal), Mithridate (polycythaemia vera), MyMelanoma (melanoma), PETReA (follicular lymphoma), Prelude (breast cancer-related lymphoedema), REMoDL-A (diffuse large B-cell lymphoma), Rudy (acute myeloid leukaemia), , UKAITPR (idiopathic thrombocytopenic purpura) and UKKCC (cutaneous squamous cell carcinoma).

BaBi Warwick is a large-scale data collection project which aims to recruit ALL women who have their babies at Warwick Hospital. The project aims to answer research questions that serve our local population to help make a difference and improve outcomes for families in our area. The research questions are to be decided by Warwick Hospital and involve the council, local charities, universities and others. BaBi Warwick is the first study of its kind in the Midlands, and we are so excited to be one of the first hospitals taking part.
BaBi Warwick will collect data from each woman who agrees to participate in the study to answer relevant research questions around our local population. This data will be kept secure and used only for research and service planning.
The SURFSUP Trial
The SURFSUP Trial (SURFactant Administration by SUPraglottic Airway) is a research study comparing two methods of giving surfactant treatment to premature babies with respiratory distress syndrome (RDS). Surfactant is a liquid medicine that helps babies breathe more effectively. The standard method involves using a laryngoscope to place a thin tube into the airway, while a newer approach uses a supraglottic airway, a flexible tube inserted without a laryngoscope. Both methods are currently used at the Children’s Hospital in Glasgow, but it is unclear which is better.
Supraglottic airways are easier to place and may reduce the number of attempts and risks compared to the standard method. Early studies with around 350 babies suggest this approach is effective, but a larger trial is needed to determine whether it should become the standard treatment. The SURFSUP Trial aims to find out if this newer method is as effective as the current standard.
OBS UK
The OBS UK Study (Clinical and Cost-Effectiveness of a Maternity Quality Improvement Programme to Reduce Excess Bleeding and Need for Transfusion After Childbirth) is investigating better ways to manage heavy bleeding, the most common complication of birth. The OBS UK study team has developed a new approach, the OBS UK care bundle, designed to recognise and treat heavy bleeding more effectively. When introduced in Wales, it reduced the need for blood transfusions and the number of severe bleeds.
This study will assess whether these benefits can be replicated across the wider NHS. If the OBS UK care bundle significantly improves outcomes, it could help shape new national guidelines for managing heavy bleeding during and after childbirth.
For more information: https:/
![]()
Perioperative Quality Improvement Programme (PQIP)
PQIP is a national research and quality improvement initiative which is looking at the perioperative care of patients having elective surgery. The aim is to investigate how these patients are treated, how they feel their care and recovery has been and if any complications have occurred. This data can then be used to improve patient care and ensure best practice is maintained throughout the whole country.
Here at Warwick Hospital, we have successfully recruited over 250 colorectal patients to the study.

ColoCap Study
The ColoCap study aims to assess the accuracy of colon capsule endoscopy (CCE) in diagnosing bowel diseases such as cancer, polyps, and inflammation by comparing it to the standard colonoscopy procedure. Colonoscopy, while highly effective, can be uncomfortable, often requiring sedation and recovery time, which may delay diagnosis due to high demand. CCE is a newer, less invasive alternative that involves swallowing a small capsule with a built-in camera, which captures images as it passes naturally through the digestive system. Patients taking part in this study will undergo both procedures on the same day, with CCE performed first, followed by a colonoscopy. The study will evaluate whether CCE can be a reliable alternative to colonoscopy, potentially improving access to timely diagnosis.
To email the Central Research Team, please email CentralResearchTeam
Plant‑Protein Dominant study

The Plant‑Protein Dominant study aims to determine whether enteral tube feeds containing mostly plant‑based proteins are as effective as standard animal‑protein dominant feeds for adults receiving home tube feeding. Enteral tube feeding is used to meet nutritional needs in people who cannot eat enough orally, and more than 40,000 adults in the UK currently receive nutrition this way. Although plant‑based diets have become increasingly popular for health, environmental, cultural and personal reasons, there is very limited research on the long‑term use of plant‑protein dominant tube feeds. Four existing formulations have recently been reformulated to contain a protein blend in which 78% of the protein is plant‑derived. In this exploratory randomised controlled trial, participants will be assigned to receive either a plant-protein-dominant feed or an animal-protein-dominant control product for three months, following a short baseline period, with an optional nine‑month extension. The study will assess gastrointestinal tolerance, compliance, acceptability, changes in body weight, and functional outcomes to determine whether plant-protein-dominant formulations can offer an effective alternative for people who rely on long‑term enteral feeding.
PRES is a survey used to collect views and experiences of individuals participating in National Institute for Health and Care Research (NIHR) supported research. Responses to PRES are kept anonymous. PRES is led by the Regional Research Delivery Network (RRDN) to help improve research experiences.
Why do we deliver PRES?
We deliver PRES surveys at SWFT to better understand the experiences of our research participants. SWFT is supported by the Regional Research Delivery Network (RRDN) who annually produce a local and regional report of the PRES findings. PRES findings from our research participants are shared back to us, giving us the opportunity to understand what we are doing well and where we can make improvements in the delivery of our research studies. Nationally the NIHR bring together all PRES responses to produce a national level report.
- Find out more about the National Institute for Health Research (NIHR).
- More information about what clinical trials are, why you should join, and what’s typically involved.
- Be Part of Research allows users to search for opportunities to participate in research.
- An online resource online resource where researchers and research organisations advertise opportunities for members of the public to get involved in research.